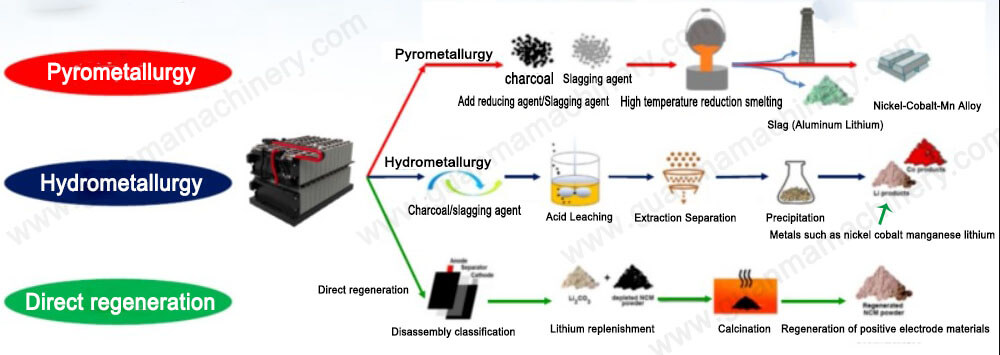
The raw materials of the gold electrolytic refining equipment are crude gold, silver electrolytic secondary black gold powder, etc., which are cast into anodes containing more than 90% gold. Electrolytic refining can produce national standard 1 or No. 2 gold.
The electrolyte for gold electrolytic refining can be a gold chloride complex aqueous solution or a cyanide complex aqueous solution, but the former is safer and widely used by various factories.
The crude gold is used as the anode, the pure gold sheet is used as the cathode, and the mixture of gold chloride complex and hydrochloric acid is used as the electrolyte.

The electrolysis process can be represented by the following electrochemical system:
Au (pure) | HCl, HAuCl₄, H₂O | Au (crude)
Chloroauric acid is a strong acid and completely ionizes in water:
HAuCl₄=H+AuCl₄
The AuCl₄ complex ion is extremely stable in solution, and the stability constant (β) of the following reaction is relatively large:
Au³+ +4Cl=AuCl₄
β₄ =2 x10*21, so it can be considered that gold exists in the form of AuCl₄ in chloride solution.