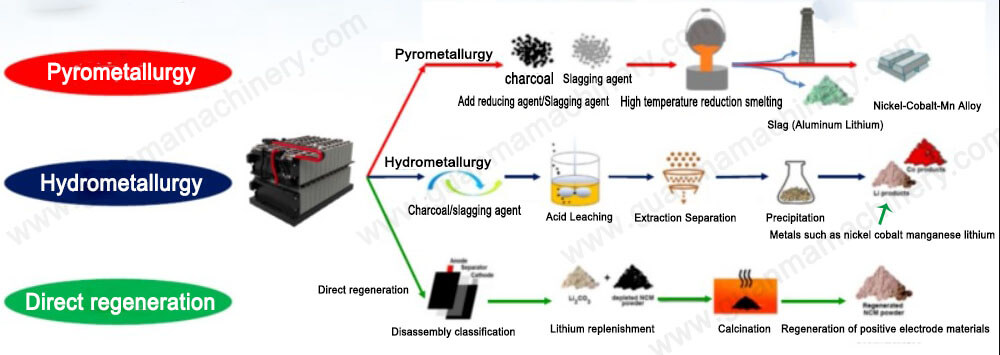
Copper electrolysis is a process that involves using electricity to refine and purify copper.
Here’s a step-by-step guide on how to perform copper electrolysis:
1. Prepare the Electrolyte Solution:
Use a solution of copper sulfate as the electrolyte. This solution will conduct the electric current and facilitate the electrochemical reactions1.
2. Set Up the Electrolytic Cell:
Place a titanium-coated anode plate and a pure copper cathode plate into the electrolytic cell, alternating them1.
Ensure the cell is properly sealed and the electrolyte solution is at the required level.

3. Apply Electric Current:
Connect the anode and cathode to a power supply that provides a direct current (DC).
Turn on the power supply to initiate the electrolysis process.
4. Monitor the Reactions:
At the anode, copper and any impurities present in the impure copper will dissolve into the electrolyte solution.
At the cathode, pure copper will be deposited as the copper ions in the electrolyte solution are reduced by the electric current.
5. Collect the Pure Copper:
Once the electrolysis process is complete, the pure copper deposited on the cathode can be collected and further processed as needed.
6. Control Parameters for Efficiency:
To prevent abnormal electrodeposition and improve the appearance of the electrolytic copper, specific parameters such as electrolyte concentration and current density should be carefully controlled.
By following these steps, copper electrolysis can be performed effectively to refine and purify copper. This process is crucial in the production of high-quality copper products.