Spent lithium-ion batteries contain valuable and non-renewable metals such as cobalt, lithium, nickel, copper, and aluminum. The Hydrometallurgical process for recycling lithium-ion batteries involves the use of aqueous solutions to separate and recover these metals. Techniques include leaching, solution purification and concentration, solvent extraction, and electrowinning to obtain pure metal products.
For spent lithium iron phosphate (LFP) batteries, a strong acid is used to dissolve the positive electrode, after which alkali is added to precipitate lithium, iron ions, and phosphate ions. The recovered materials are then adjusted according to the desired ratio and thermally treated to produce regenerated LFP.
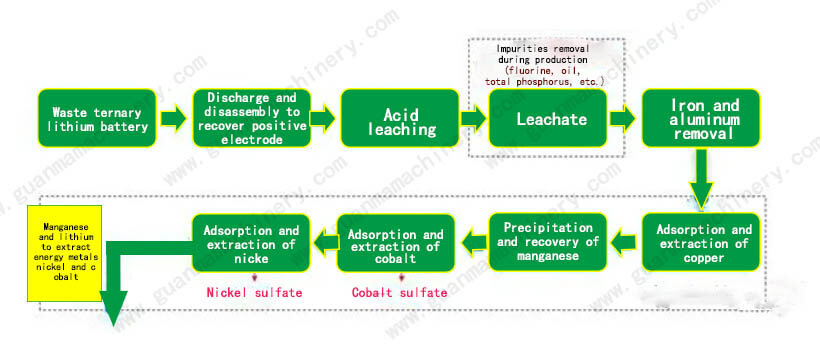
Key Steps in the Hydrometallurgical Process:
1. Dissolution: Metal ions are dissolved using acid and base solutions, typically sulfuric acid (H₂SO₄), sodium hydroxide (NaOH), and hydrogen peroxide (H₂O₂).
2. Precipitation and Adsorption: Dissolved metal ions are extracted as oxides or salts through precipitation and adsorption processes.
3. Purification and Concentration: Solutions are purified and concentrated to remove impurities and increase the concentration of target metals.
4. Solvent Extraction: Copper is separated from cobalt and nickel using solvent extraction.
5. Electrowinning: Copper, cobalt, and nickel are recovered through electrowinning to produce high-purity metal products.
Process for Recycling LFP Batteries:
Aluminum Foil Separation: Prior to the wet process, the aluminum foil current collector is separated from the active material of the positive electrode.
Pre-Treatment: The spent batteries undergo discharge, disassembly, crushing, and sorting.
Plastic and Steel Recovery: The plastic and steel casings are recovered separately.
Leaching: The active materials are subjected to alkaline and acidic leaching.
Purification and Concentration: Leached solutions are purified and concentrated to remove impurities and increase the concentration of target metals.
Solvent Extraction: Copper is separated from cobalt and nickel.
Electrowinning: Copper, cobalt, and nickel are recovered through electrowinning to produce high-purity metal products.
Product Formation: After solvent extraction, cobalt and nickel can either be crystallized or electrowon to produce cobalt and nickel salts or metals.
GuanMa Machinery’s hydrometallurgical recycling technology for lithium-ion batteries follows these steps:
1. Pre-Processing: Spent batteries are discharged, disassembled, crushed, and sorted.
2. Material Recovery: Plastic and steel casings are recovered.
3. Leaching: Active materials are leached using alkaline and acidic solutions.
4. Solvent Extraction: Copper is separated from cobalt and nickel.
5. Electrowinning: Copper, cobalt, and nickel are recovered through electrowinning to produce high-purity metal products.
The recovery rate for copper, cobalt, and nickel through electrowinning reaches 99%, with product qualities reaching 99.98% for cobalt, 99.95% for copper, and 99.2% to 99.9% for nickel. The produced cobalt sulfate and nickel sulfate meet relevant standards.




